I heard this phrase during a presentation on rare/orphan diseases at the recent AMCP Annual Meeting in Boston. Pharmacists from a payer organization reviewed three rare diseases, products currently available to treat them and the pipeline of products in development. Part of the discussion focused on payer sensitivity to the cost of these products and the need to limit their use to patients most likely to benefit – because payers are the “stewards of the resources”.
This was the first time I had heard payers describe themselves in this manner…interesting to say the least.
Payers are the gatekeepers
There is no denying the pivotal role that payers play in the accessibility of medications for patients. They are the gatekeepers who determine what products will be covered and at what level of out-of-pocket cost to the patients. While manufacturers of products that treat the larger disease states like ADHD or hyperlipidemia are familiar with this dynamic, manufacturers in the rare/orphan space may not be. Historically, products for rare/orphan diseases faced few hurdles with payers for coverage. The availability of treatments for such conditions were in themselves rare.
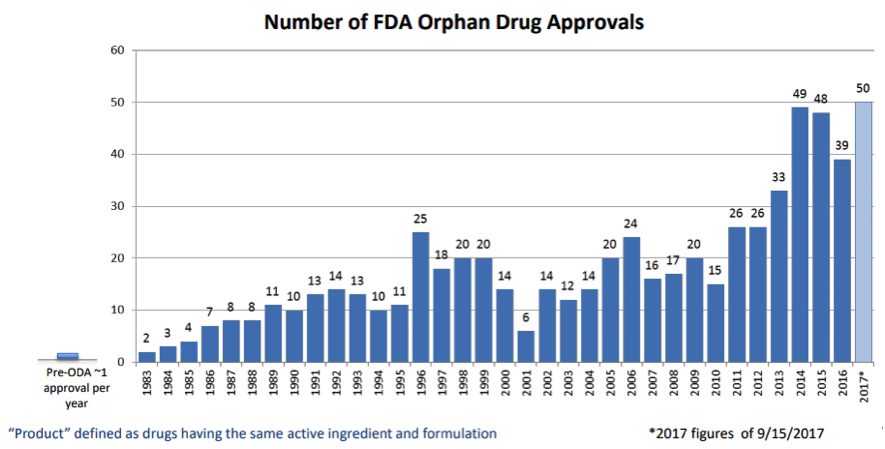
Today, however, times have changed. Many more players have entered the space, and the FDA’s approval of such products has increased greatly especially in the last five years, as demonstrated by data reported in the FDA’s Insights into Rare Disease Drug Approvals.
Given the potential impact collectively on payer budgets, rare/orphan disease companies cannot take for granted that coverage or reimbursement for their product will come easy. The presentation at AMCP reinforced that message.
Be Aware and Be Prepared
Payers manage resource use by limiting access to products. For expensive orphan/rare disease products, payers want to limit their initial use to those most likely to respond. They also want to be able to discontinue use in patients who ultimately are not benefiting. This is where things get interesting.
Payers seem to start with the clinical trial inclusion criteria as the first pass at coverage criteria. In their mind, those were the patient types who ultimately responded well enough for the FDA to approve the product. Once a patient is approved for coverage, they worry about its indefinite use. In their view, they want some way to measure response to be able to “advocate for discontinuation” as the speaker said. Knowing if the medication is working is important.
If you’re in the rare/orphan space, take a look at your trial inclusion criteria and medication response measures – payers certainly are. Those factors can quickly become the crux of market access negotiations that impact commercial success or struggle of your product.
Viking Healthcare Solutions has established itself as the premier provider of corporate account services and strategic planning support for the pharmaceutical and biotech industries. VHS Insights, our research division, specializing in payer profiling, market research and analytics, can help you find answers to inform your payer strategy. We support traditional and rare/specialty products to create, maintain, defend, and protect access to your product throughout its lifecycle. Bank on our experience to help you achieve successful product commercialization. Contact us at www.vikinghcs.com/connect.